gas volumetric analysis|gas volume and stoichiometry : vendor The composition of fuel gases and combustion products can be determined by measuring the changes in volume that occur when the sample is treated successively with . O CopySpider é uma ferramenta que permite detectar plágio em trabalhos acadêmicos e pode ser baixada em computadores Windows gratuitamente. Canaltech - Notícias de Tecnologia, Ciência e .
{plog:ftitle_list}
A CSGO gambling site bonus code is a promotional code offered by some online betting sites associated with the popular game Counter-Strike: Global Offensive (CSGO). These codes are often used to attract new players or reward existing ones. By entering a bonus code when registering or depositing on a CSGO gambling site, the player can receive .
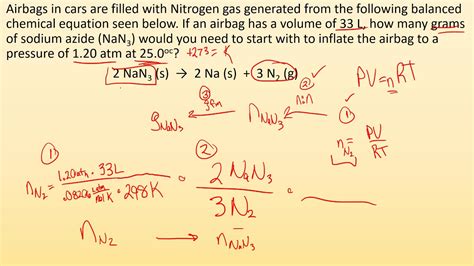
volume of gas in chemical reaction
13-46 The volumetric analysis of a mixture of gases is given. The volumetric and mass flow rates are to be determined using three methods. Properties The molar masses of O2, N2, CO2, and CH4 are 32.0, 28.0, 44.0, and 16.0 kg/kmol, respectively (Table A-1).Volumetric analysis is a technique that employs geological observations and information to estimate original fluids-in-place. It is often referred to as a "static method” as it primarily sources its data from core samples, wireline logs, and .An ideal-gas mixture has the following volumetric analysis Component % by Volume N2 60 CO2 40 (a) Find the analysis on a mass basis.The volume of gas produced during a chemical reaction can be measured by collecting the gas in an inverted container filled with water. The gas forces water out of the container, and the volume of liquid displaced is a measure of the .
The composition of fuel gases and combustion products can be determined by measuring the changes in volume that occur when the sample is treated successively with .Volumetric analysis is a quantitative analytical method which is used widely. As the name suggests, this method involves measurement of the volume of a solution whose concentration is known and applied to determine the .Apply the rules for determining mixture properties to ideal-gas mixtures and real-gas mixtures. Predict the P-v-T behavior of gas mixtures based on Dalton’s law of additive pressures and .
Following a synopsis of the techniques involved, a discussion of the main equipment used and the key features of primary and secondary standards is presented. . In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of multicomponent gas mixtures, which are . Volumetric analysis is also referred to as volumetry —this term makes it much easier to see the principle behind this method of analysis: A solution of a reagent is added to a .Experiment 7 Postlab expt gas volumetric analysis: the concentration of hydrogen peroxide solution calculations and results: 30. 03 𝑖𝑛𝐻𝑔 762. 𝑚𝑚𝐻𝑔 004 𝑎𝑡𝑚 20
Volumetric gas analysis means that the overall gas volume has been measured beforehand. When the overall gas volume changes because of the chemical reaction of one individual component, the volume of the individual component results from the difference in the overall gas volume before and after the reaction. A similar system is used for . Evaluation of natural gas hydrate resources in the South China Sea by combining volumetric and trend-analysis methods. December 2021; Petroleum Science 19(1) . and the gas volume . 320.A certain natural gas has the following volumetric analysis (mole fractions): 65 percent CH4,8 percent H2,18 percent N2,3 percent O2, and 6 percent CO2. This gas is now burned completely with the stoichiometric amount of dry air.
A furnace burns natural gas with a volumetric analysis as follows: Methane = 85%, Ethane = 12%, Propane = 3%. The gas flow rate is 0.50 m3/s and 25% excess air is required for complete combustion. Combustion air is supplied to the furnace at 25oC and 1atm pressure. Find the molal air fuel-ratio and the volume flow rate of the flue gas in m^3/s.A fuel gas has the following volumetric analysis: CH4=68.0 C2H6=32.0 assume complete combustion with 15% excess air at 14.7 psia, wet-bulb 70 degrees F and dry bulb 80 degrees F determine the following: a) volume of actual (dry + water vapor) air required per 1000 ft^3 of gas at same pressure and temperature b) water dew-point temperature of .
Lab 4: Gas Volumetric Analysis: The Concentration of a Hydrogen Peroxide Solution. Flashcards; Learn; Test; Match; Q-Chat; Get a hint. objective. Click the card to flip 👆. determine the concentration of hydrogen peroxide volumetrically through application of the ideal gas law to the volume of gas that is evolved when we decompose the .
A high-performance H2 gas sensor system based on capacitive electrodes and a volumetric analysis technique were developed. Coaxial capacitive electrodes were fabricated by placing a thin copper .
Gas 0/ C] The pressure-volume-temperature behavior of a single-compound hydro- carbon showing the temperature and pressure conditions that produce a gas or liquid phase. At the pressure and temperature corresponding to the criti- cal point, the gas and liquid phases are indistinguishable. Cell All Liquid First Gas Bubble Gas and Liquid Last Droplet
A fuel gas has the following volumetric analysis: CH4 = 68% C2H6 =32% Assume complete combustion with 15 % excess air at 101.325 kPa, 21oC wet bulb and 27oC. Determine the actual fuel ratio. What is the partial pressure of the water vapor?A gas mixture consists of 20 percent O2, 30 percent N2, and 50 percent CO2 on mass basis. Determine the volumetric analysis of the mixture and the apparent gas constant. The universal gas constant is Ru= 8.314 kJ/kmol-K. Use the table containing the molar mass, gas constant, and critical-point properties. The volume fraction of O2 is %.Volumetric analysis through the application of the ideal gas law to the volume of gas evolved when we decompose H2O2 What is the chemical reaction H2O2(l) -> H2O (l) + 1/2 O2(g)An ideal-gas mixture has the following volumetric analysis Component % by Volume N2 60 CO2 40 (a) Find the analysis on a mass basis. Chapter 12-9 For ideal-gas mixtures, the percent by volume is the volume fraction. Recall yvfii= Comp. .
Volumetric analysis Volumetric analysis is also known as titrimetric analysis. The reagent (the titrant) is added gradually or stepwise to the analyte from a burette. The key to performing a successful titrimetric analysis is to recognize the equivalence point of the titration (the point at which the quantities of the two reacting species are equivalent), typically observed as
volume fraction model
The Development of the volumetric technique for measuring gas adsorption equilibria is date back to the early of 20th century, by Sieverts, which considered a glass volumetric apparatus for absorption and diffusion of gases [11].This early volumetric apparatus passed it’s evolution and was improved by pressure measurement, temperature control and . Of this volume of natural gas, 0.85 trillion m3 has already been extracted, and the approved geological reserves are estimated at 2.55 trillion m3 . Almost 83% of the extracted natural gas .
From volumetric analysis of gas we can determine the minimum quantity of air required, the total volume of products of combustion and water vapour formed. When the steam has been condensed, the volume of the dry products of combustion can be calculated. When the exhaust gases are analysed the steam will be condensed. Original Gas-in-place refers to the total volume of gas stored in a reservoir prior to production. Metric Calculation: Variables: Rock Volume (m 3) = 10 4 * A * h; . calculations and is dependent on the limitations of each parameter in the equation is .11 Gas Laws Notes. Notes. 12 Liquids and Solids Notes. Notes. 13 Solution Chemistry Notes. Notes. Collapse 14 Volumetric Analysis 14.1. Introduction 14.1. Introduction. 14.2. . Volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. It uses titration to determine the concentration of a . Key Concepts. Arrhenius Acid: A substance that yields hydrogen ions (H +) when dissolved in water.; Arrhenius Base: A substance that yields hydroxide ions (OH-) when dissolved in water. Bronsted acid : A substance capable of donationg a proton.Bronsted base: A substance capable of accepting a proton. Chemical Equilibrium: A state in which the rates of the forward .
Gas Volumetric Analysis: The Concentration of a Hydrogen Peroxide Solution Chemistry 105a – Fall 2015 Trial # 3: P O 2 = P total − P water 759.0torr - 2.64torr P O2 = 756.36 torr 1 atm 760 torr P O2 = 0.995 atm N O 2 = P O 2 ×ΔV O 2 RT (0.995 atm)(0.0496 L − 0.0161 L) (0.08206 atm∙ L mol∙K )(295.05 K) 1.38x10-3 molO 2 1.38 x 10 − .Determine the concentration of a solution of H2O2 using volumetric analysis and the ideal gas law. What does H2O2 decompose into? H2O + 1/2 O2. Is the decomposition of liquid hydrogen exothermic or endothermic? Very exothermic. If a reaction releases a lot of heat, is it .A furnace burns natural gas with a volumetric analysis as follows: Methane (CH 4)= 80%, Ethane (C 2 H 6) = 15%, Propane (C 3 H 8)= 5%. 18% excess air is supplied for complete combustion.Find the stoichiometric molal air-fuel ratio.A furnace burns natural gas with a volumetric analysis as follows: Methane (CH4)= 80%, Ethane (C2H6) = 15%, Propane .
Post Lab 4: Gas Volumetric Analysis Completed Data Table: Trial Temp (K) Pressure (atm) 1 294 1. 2 294 1. 3 294 1. Trial % Content Hydrogen Peroxide. Standard Deviation of % Concentration 1 2 0. 2 2. 3 2. Average: 2. Trial. The volumetric analysis of gaseous reaction using eudiometric tube called Eudiometry or “Volume analysis of gas” In eudiometric tube all the measurement of volume is done at constant pressure and temperature and given gaseous reaction at .The Volumetric Method for OGIP is essentially the same for gas reservoirs. The method uses static geologic data to determine the volume of the pore space of the reservoir. Once the volume of the pore space is estimated, then the gas formation volume factor, B g can be used to estimate the OGIP. By simply using the definition of reservoir volumes, the original-gas-in-place of the .
A natural gas with the volumetric analysis 94.2% methane (CH4), 4.9% ethane (C2H6), 0.9% nitrogen (N2) is burned with air in a furnace to give products having a dry molar analysis of 10.21% CO2, 86.78% N2, and the remainder 02. Determine: a. the stoichiometric air-fuel ratio on a molar basis b. the percentage excess air for the actual combustion.
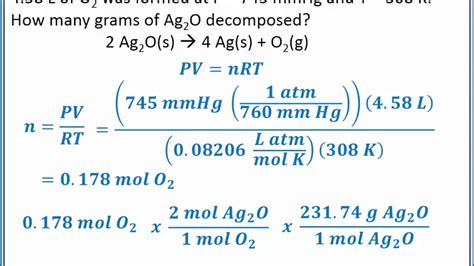
scratch test ctev
WEB14 de dez. de 2018 · Enjoy the entire thirteenth season of the PBS television series: Bob Ross - The Joy of Painting. #HappyTrees #ThankYouBob #OilPainting #BobRoss #TheJoyOfPain.
gas volumetric analysis|gas volume and stoichiometry